|
In R3 the spherical harmonics correspond to the harmonic
poylnomials that are homogeneous of degree l; we have
dim(Hd) = 2l+1 = 1,3,5,7,...
The pure states of a hydrogen atom are given by its principal quantum number
N=1,2,3,... its angular momentum l, and its magnetic quantum number m.
The energy in state N is -1/N2.
Together l and m pick out a basis element Ylm from the
space Hl.
These satisfy
-l &le m &le l and 0 &le l &le N-1.
The wave function has the form
f(x,y,z) = e-r rl LNl (r) Y lm (x,y,z),
where the radial function LNl(r) is a Laguerre polynomial.
Thus the number of states of hydrogen with energy N is given by
dim(H0) + ... + dim(HN-1) = n2.
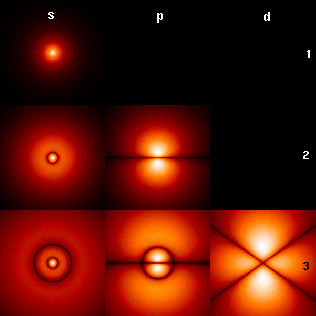
Traditionally states with values l=0,1,2,3,... are denoted by
s, p, d, f, g, h. Note that a given spherical harmonic in Hl
appears for all energies N>l.
(Disclaimer: We ignore the fine structure constant.)
|